세포주은행 제조
(주)유바이오로직스가 제공하는 안정적인 세포주은행 제조서비스는 정확한 특성분석을 거쳐 균일한 조성의 세포 내용물로 구성된 세포주은행 구축을 지원합니다.
Cell Bank Production (MCB/WCB)CRMO
Contract R&D and Manufacturing Organization(주)유바이오로직스의 수탁생산 서비스는 백신, 유전자재조합 치료제 및 다양한 바이오 의약품에 대해 선진 GMP에 부합되도록 설계되었습니다.
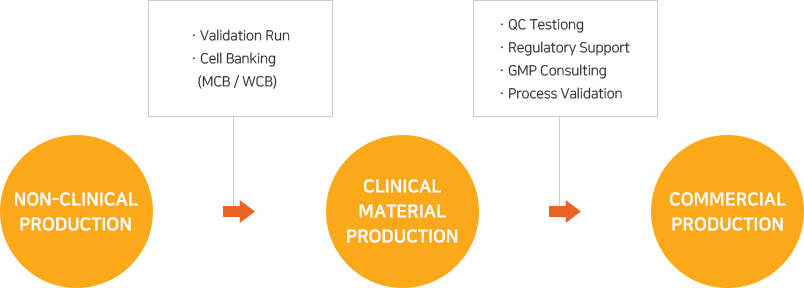
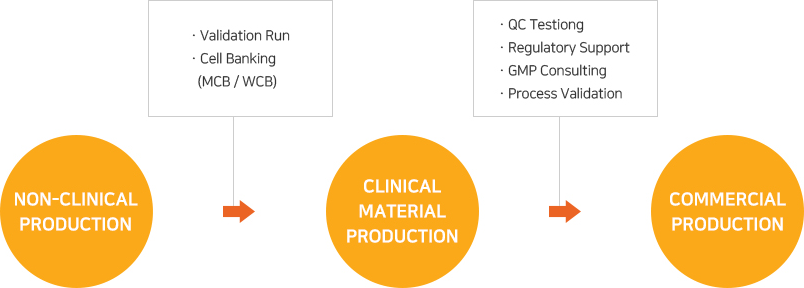
(주)유바이오로직스의 수탁생산 서비스는 백신, 유전자 재조합 치료제, 항체치료제 등 다양한 바이오 의약품에 대해 선진 GMP에 부합되도록 설계ㆍ검증된 생산시스템 및 품질관리시스템을 바탕으로 제공됩니다. 해외 진출을 고려하는 고객의 경우에도 국가별 GMP 운영 및 실사에 대한 부담 없이 프로젝트를 진행할 수 있습니다.
초기 임상 단계부터 상업생산에 이르기까지 국내 최고의 경험을 보유한 전문인력이 각 고객의 목표 및 개발단계에 부합되는 서비스를 제공합니다.
특히 프로젝트 진행 중 발생되는 문제점에 대해서도 함께 고민하고 해결책을 찾는데 큰 도움이 되어 드릴 것입니다.

(주)유바이오로직스가 제공하는 안정적인 세포주은행 제조서비스는 정확한 특성분석을 거쳐 균일한 조성의 세포 내용물로 구성된 세포주은행 구축을 지원합니다.
Cell Bank Production (MCB/WCB)
(주)유바이오로직스는 동물세포배양과 미생물발효를 이용한 두개의 배양 시스템과 최신의 기술을 적용하는 고순도 정제공정 을 이용하여 바이오의약품의 원료의약품 생산서비스를 제공합니다.
Biopharmaceutical APIs Production
(주)유바이오로직스는 무균조제 및 충전시설을 이용하여 액상 바이알, 동결건조형 바이알, 액상 튜브 등 여러 가지 형태의 완제생산서비스도 제공합니다.
Finished Product Production