Cell Bank Production
The reliable Cell Bank Production Service provided by EuBiologics supports the establishment of cell lines consisting of homogeneous cell contents through exact characteristic analysis.
Cell Bank Production (MCB/WCB)CRMO
Contract R&D and Manufacturing OrganizationEuBiologics’ CMO services are designed to meet the advanced GMPs on vaccines, recombinant protein products and various biomedicines.
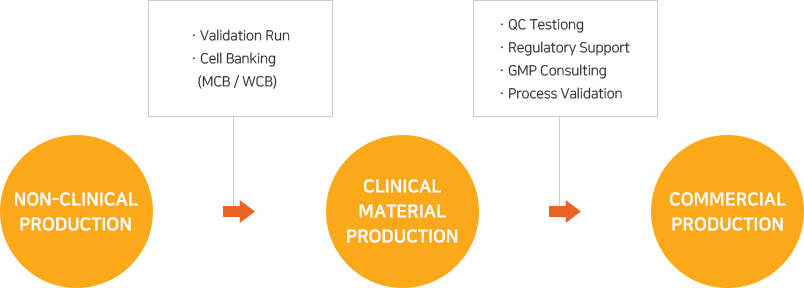
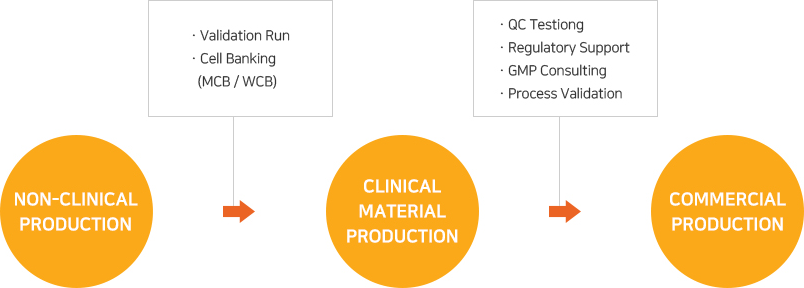
As EuBiologics’ CMO services are offered based on production systems and quality management systems designed and constructed to meet the advanced GMPs covering various biomedicines including vaccines, recombinant protein products and therapeutic antibodies, any customer contemplating overseas expansion may proceed with its project with EuBiologics without regard to local GMP regulatory inspection.
From early clinical stages to commercial production, top experts in the country will provide services that meet the customer’s goals in each development stage and will discover and address any issues that may arise during the project’s progress.

The reliable Cell Bank Production Service provided by EuBiologics supports the establishment of cell lines consisting of homogeneous cell contents through exact characteristic analysis.
Cell Bank Production (MCB/WCB)
EuBiologics provides the production services for drug substance of biomedicines by adopting the high purity purification process based on various technologies and the systems of animal cell culture and microbial fermentation.
Biopharmaceutical APIs Production
EuBiologics provides various types of drug product production services by using aseptic formulation and filling facilities for liquid vials, lyophilized vials and liquid tubes.
Finished Product Production